Introduction: The Hydrochemical Singularity of the Southeast Valley
Table of Contents
ToggleThe maintenance of recreational aquatic environments—specifically residential and commercial swimming pools—within the geographic confines of Queen Creek and San Tan Valley, Arizona, represents a unique hydrochemical challenge that diverges significantly from standard pool management practices observed in temperate North American climates. While the fundamental principles of water chemistry remain constant, the environmental variables present in this specific region of the Sonoran Desert create a “hydrochemical singularity.” Here, the convergence of extreme ambient thermal loads, exceptionally high evaporation rates, geologically distinct source water profiles, and the atmospheric deposition of agricultural byproducts necessitates a sophisticated, almost clinical approach to water management.
For the homeowner or facility manager in Queen Creek, the swimming pool is not merely a static vessel of water but a dynamic chemical reactor exposed to one of the harshest environments on the continent. The region’s rapid population growth has placed thousands of new pools into an environment where the traditional “test and treat” methodologies—often sufficient for pools in the Midwest or Pacific Northwest—are demonstrably inadequate. The distinct chemistry of the local aquifer, characterized by naturally high calcium hardness and total alkalinity, interacts with the arid climate to create a water profile that is naturally scale-forming and resistant to pH stabilization.1 Furthermore, the proximity of these residential zones to active agricultural land introduces a biological variable—nutrient-laden dust—that accelerates eutrophication (algae growth) at rates that defy standard sanitation protocols.
This report provides an exhaustive analysis of these factors, dissecting the intricate relationship between the desert environment and pool chemistry. It aims to move beyond generic advice and establish a region-specific standard of care that prioritizes the Langelier Saturation Index (LSI), strict control of Total Dissolved Solids (TDS), and the strategic deployment of specialized chemical agents to counteract the specific threats posed by the local ecosystem. The objective is to define the “best” pool chemicals not by brand preference, but by their chemical suitability for the rigorous demands of the Arizona summer.
Section 1: Understanding Arizona’s Water Profile
To effectively manage a swimming pool in Queen Creek or San Tan Valley, one must first understand the raw material: the water itself. Unlike regions that rely on soft surface water reservoirs, the Southeast Valley sits atop complex aquifers and relies on a blend of groundwater and surface water transported via the Central Arizona Project (CAP). This blend results in a baseline water profile that is fundamentally distinct in its mineralogy, hardness, and contaminant load.
1.1 The Hardness Matrix: Calcium and Magnesium Dynamics
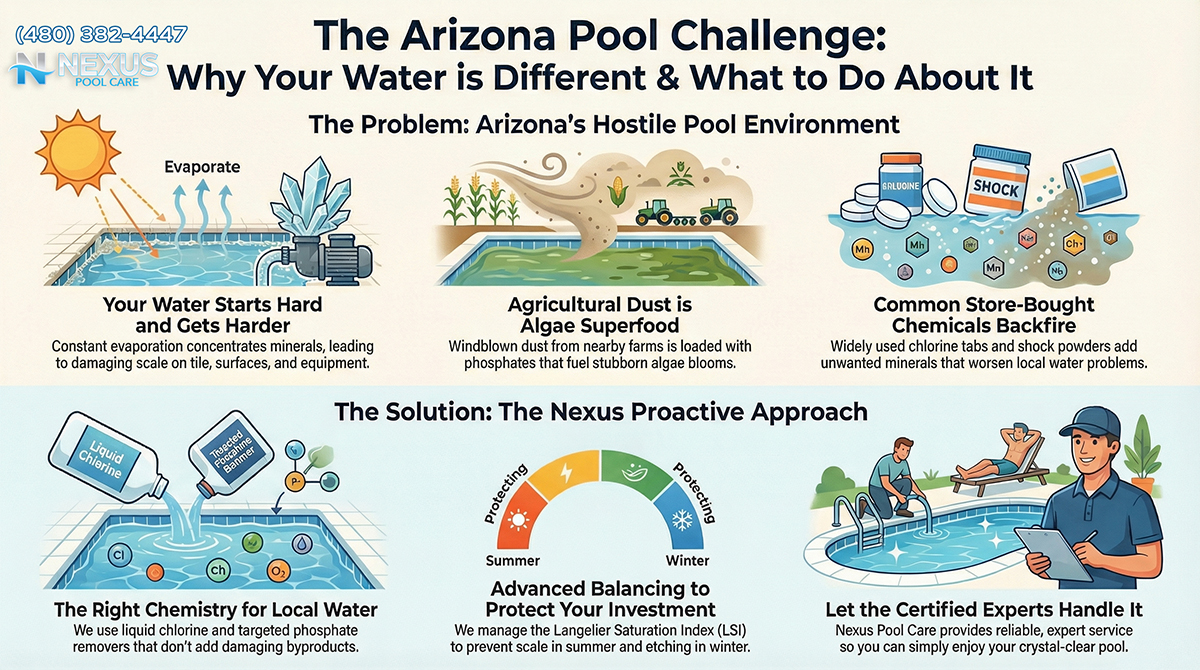
Water hardness, defined as the concentration of dissolved multivalent cations—primarily calcium ($Ca^{2+}$) and magnesium ($Mg^{2+}$)—is the defining characteristic of the local water supply. According to the Town of Queen Creek’s Annual Water Quality Reports, the local water supply exhibits hardness levels ranging significantly, often between 6 and 17 grains per gallon (gpg).1 In the lexicon of pool chemistry, where 1 grain per gallon is equivalent to approximately 17.1 parts per million (ppm), a hardness level of 17 gpg translates to nearly 291 ppm of calcium hardness straight from the tap.
This initial value is critical because the accepted industry standard for calcium hardness in a plaster or pebble-tec pool is typically 200 to 400 ppm. Consequently, a pool in Queen Creek filled with fresh municipal water begins its life at the upper threshold of the ideal range. This leaves virtually no buffer for the natural accumulation of calcium that occurs over time. In contrast to other regions where pool owners must purchase calcium chloride flakes to raise hardness levels to protect the plaster surface, the Arizona pool owner is fighting a defensive battle against calcium saturation from day one.
The implications of this high baseline are profound. Calcium is a stable mineral; it does not degrade in sunlight, nor does it evaporate. As water leaves the pool through evaporation—a process that removes pure $H_2O$ exclusively—the calcium remains behind. When this lost water is replaced by the automatic leveler (autofill) with more calcium-rich source water, the concentration of calcium in the pool rises inexorably. This “ratchet effect” means that without significant water exchange (draining), a pool in San Tan Valley can easily exceed 1,000 ppm of calcium hardness within two to three years of operation.7 At these levels, the water becomes aggressively scale-forming, precipitating calcium carbonate ($CaCO_3$) onto spillways, tile lines, and crucially, inside the heat exchangers of pool heaters and the electrolytic cells of salt chlorine generators, leading to catastrophic equipment efficiency loss.
1.2 The Alkalinity and pH Drift Phenomenon
Closely linked to hardness is the issue of Total Alkalinity (TA) and pH. The source water in the region typically enters the home with a pH range of 7.4 to 8.2 and elevated alkalinity. In the context of pool chemistry, alkalinity acts as a buffer for pH, resisting changes in acidity. However, the specific geological makeup of the aquifer contributes to a water profile that has a strong natural tendency to rise in pH.
This “pH drift” is exacerbated by the desert environment. As water is aerated—whether through the use of water features, spillways, or simply the disturbance caused by swimmers—carbon dioxide ($CO_2$) dissolved in the water off-gasses into the atmosphere. According to Henry’s Law, as $CO_2$ leaves the solution, the pH of the water rises. In Arizona, where pools often feature negative-edge spillways or aeration features to cool the water, this off-gassing is accelerated. The result is a pool that perpetually seeks a pH of 8.0 or higher.
The synergy between high calcium hardness and high pH is destructive. The solubility of calcium carbonate decreases as pH rises. Therefore, the naturally high pH of Queen Creek pools acts as a catalyst for scaling, forcing the abundant calcium out of solution and onto pool surfaces. This necessitates a chemical maintenance strategy that is heavily focused on acid demand—using significant quantities of muriatic acid not just to lower pH for swimmer comfort, but to keep the water sufficiently aggressive to hold its mineral load in solution.
1.3 Total Dissolved Solids (TDS) and Mineral Heavy Loads
Beyond calcium and magnesium, the “mineral heavy” nature of the water includes a spectrum of other dissolved solids, collectively measured as TDS. The Central Arizona Project (CAP) water, which supplements the groundwater supply, is derived from the Colorado River. This water has traversed distinct geological formations, picking up salts, sulfates, and silicates along its journey.
High TDS is often referred to as “tired water” in the industry. As TDS levels rise—compounded by the addition of pool chemicals like liquid chlorine (which adds sodium and chlorides), acid (which adds chlorides or sulfates), and shock (which adds calcium or cyanuric acid)—the efficacy of sanitizers diminishes. High TDS interferes with the transmission of light, making water appear dull or flat even when chemically balanced, and can affect the refractive index of the water. Furthermore, elevated TDS levels increase the conductivity of the water, which can accelerate galvanic corrosion of metal components in the pool, such as light niches, ladders, and heater cores.
In Queen Creek, the rate of TDS accumulation is significantly higher than the national average due to the high evaporation-refill cycle. A standard 15,000-gallon pool in this region may lose upwards of 25,000 gallons of water to evaporation annually. This means the entire volume of the pool is effectively “distilled” and reconstituted with mineral-laden water nearly twice over in a single year, importing hundreds of pounds of additional dissolved solids that have nowhere to go but into the solution.
1.4 Trace Contaminants: The Arsenic and Nitrate Factor
While municipal water quality reports confirm compliance with federal safety standards, they also reveal the presence of trace contaminants that, while safe for human consumption, complicate pool chemistry. Reports for the region indicate the presence of arsenic (ranging up to 7.6 parts per billion) and naturally occurring fluorides. While arsenic at these levels poses negligible health risks to swimmers, its presence is a marker of the complex metalloid profile of the water.
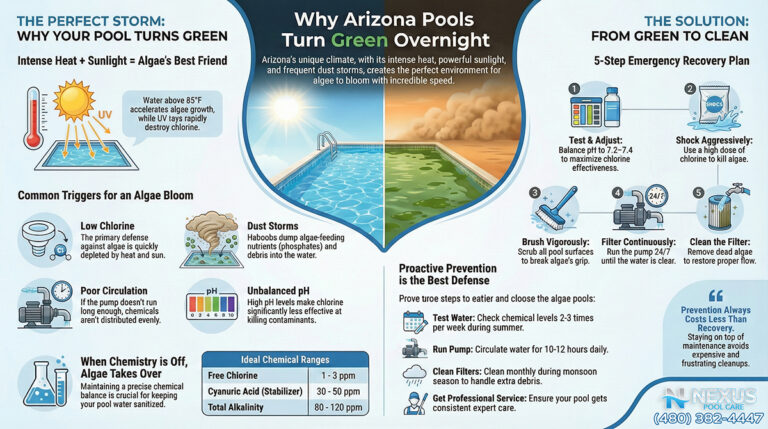
More concerning for pool management is the presence of nitrates. Sourced from both natural geological deposits and the legacy of agricultural fertilization in the San Tan Valley area, nitrates are a primary nutrient for algae. Even low levels of nitrates in source water (the EPA limit is 10 ppm for drinking water) can provide a base level of nutrition for algae, meaning the water arrives “pre-fertilized”. This creates a “chlorine demand” from the moment the pool is filled, as the sanitizer must work to oxidize these organic nitrogen compounds alongside its primary duty of pathogen control.
The combination of these factors—high initial hardness, a propensity for pH rise, rapid TDS accumulation, and a baseline nutrient load—defines the “Arizona Water Profile.” It is a profile that is chemically stubborn, prone to scaling, and biologically supportive of algae growth. Consequently, the selection of pool chemicals cannot be generic; it must be specifically targeted to neutralize these inherent disadvantages.
Section 2: Essential Chemicals Every Arizona Pool Needs
Given the aggressive and mineral-rich nature of the water profile established above, the chemical pharmacopeia for a Queen Creek pool differs in priority and quantity from pools in other climates. The following analysis details the essential chemical categories required for maintaining sanitary and structural integrity in this environment, distinguishing between “convenience” products and “essential” tools.
2.1 Sanitizers: The Liquid Chlorine Imperative
The selection of a primary sanitizer is the single most critical decision for a desert pool owner. While the market offers various delivery mechanisms for chlorine, the specific conditions of the Arizona summer render some of them detrimental.
The Trichlor Tablet Paradox
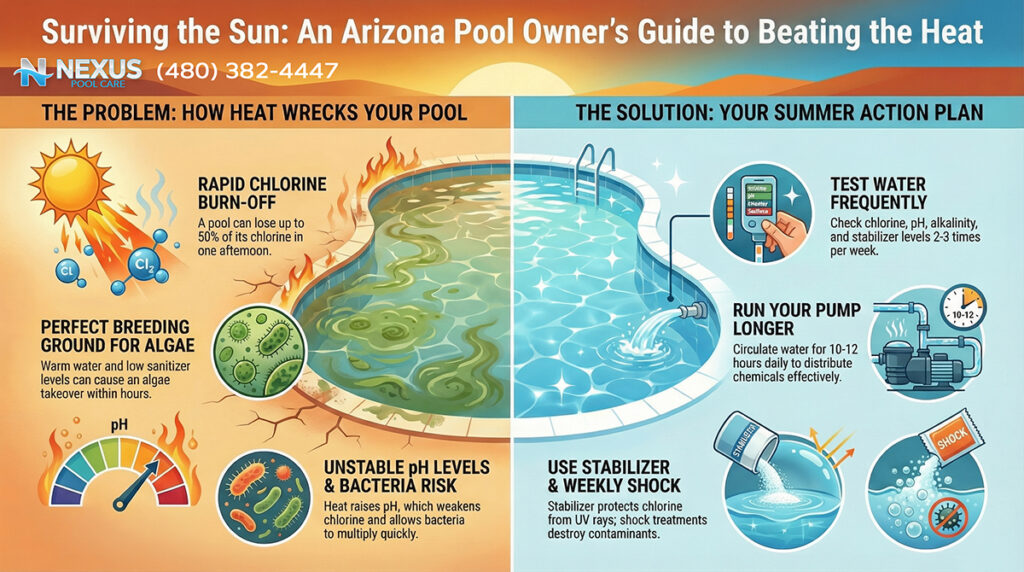
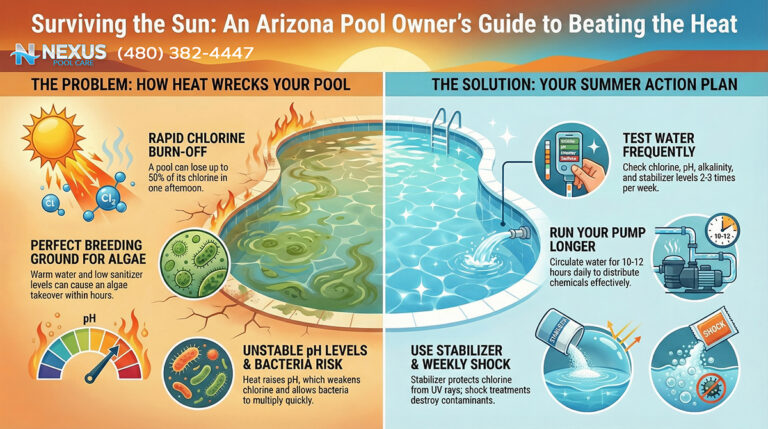
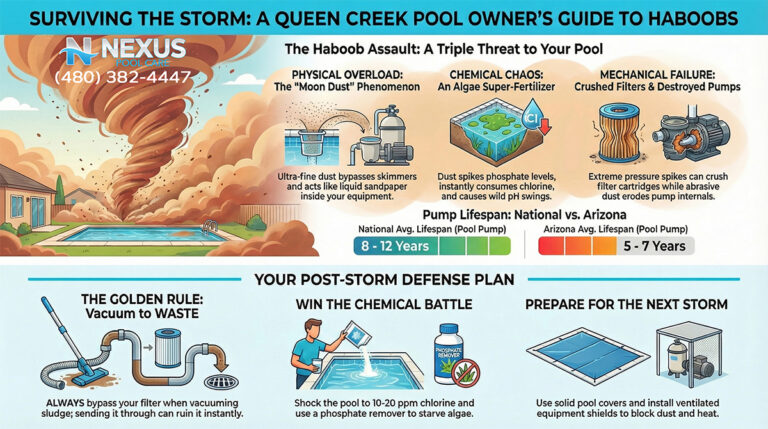
Trichloro-s-triazinetrione (commonly known as “tabs” or “pucks”) is the most widely used sanitizer due to its convenience. However, in the Arizona desert, it functions as a “Trojan Horse.” Trichlor is composed of approximately 50% Cyanuric Acid (CYA) by weight. CYA acts as a stabilizer, protecting chlorine from UV degradation. In moderation (30–50 ppm), CYA is essential. However, because Trichlor adds CYA with every gram of chlorine, and because Arizona pools require high chlorine throughput due to heat and UV, the use of tabs leads to a rapid and uncontrollable rise in CYA levels.
When CYA levels exceed 100 ppm—a common occurrence in tab-managed Arizona pools—the efficacy of the chlorine is severely retarded. This phenomenon, often termed “chlorine lock” or over-stabilization, means that even high levels of Free Chlorine (FC) are chemically bound to the stabilizer and unavailable to kill algae. This leads to the baffling scenario where a pool owner tests their water, sees “high chlorine,” yet watches their pool turn green. The only remedy for high CYA is draining the pool, which is economically and environmentally painful in a drought-prone state.
Liquid Chlorine (Sodium Hypochlorite): The Desert Standard
For these reasons, Liquid Chlorine (Sodium Hypochlorite) is the gold standard for Arizona pool sanitation. Unlike tabs, liquid chlorine adds no CYA and no calcium to the water. It is a pure oxidizer and sanitizer that leaves behind only a small amount of salt (sodium chloride) as a byproduct.
- Mechanism: Liquid chlorine provides an immediate spike in Hypochlorous Acid (HOCl), the killing form of chlorine, without the “slow release” lag of tablets. This is crucial for overcoming the rapid algae growth rates seen in water temperatures exceeding 90°F.
- Concentration: In the Valley, liquid chlorine is typically sold in higher concentrations (10% to 12.5%) compared to standard laundry bleach (6%). Specialized pool retailers and hardware stores in the region often stock 12.5% strength, which is more cost-effective for the volume of water being treated.
- Application: The “Trouble Free Pool” methodology, widely advocated for hard water regions, emphasizes the exclusive use of liquid chlorine to decouple chlorination from stabilization. This allows the pool owner to lock their CYA level at the ideal range (40–50 ppm) and never worry about it creeping up, thus avoiding the need for frequent draining.
Salt Chlorine Generators: The Manufacturing Plant
Salt Chlorine Generators (SWGs) are highly recommended for Queen Creek and San Tan Valley. An SWG is essentially a miniature chlorine factory installed in the pool’s plumbing.
- Synergy with Hard Water: While SWGs are effective, they require vigilance in hard water areas. The electrolytic plates inside the cell generate heat and high pH, which attracts calcium. This leads to rapid scale formation inside the cell, reducing its life and efficiency. Arizona pool owners with SWGs must acid-wash their cells regularly (every 3 months) or use vinegar solutions to dissolve the calcium without damaging the ruthenium oxide coating on the plates.
- Consistency: The primary benefit in the desert is consistency. An SWG provides a steady trickle of chlorine all day, maintaining a residual even during the peak sun hours of 2:00 PM to 4:00 PM, whereas liquid chlorine dumped in the morning may degrade by afternoon.
2.2 Stabilizer (Cyanuric Acid): The Chemical Sunscreen
Arizona’s UV index is consistently among the highest in the world. Without Cyanuric Acid (CYA), UV radiation can destroy 90% of free chlorine within two hours.2 Therefore, CYA is an essential chemical, but it must be managed with surgical precision.
- The Arizona Range: While northern climates recommend 30 ppm of CYA, the intensity of the Sonoran sun often necessitates a slightly higher range of 40–50 ppm, and sometimes up to 60 ppm in salt pools, to hold a residual throughout the day.
- The Ceiling: The absolute limit is generally considered 80 ppm. Above this, the oxidation reduction potential (ORP) of the water drops significantly, making the chlorine sluggish against pathogens. The goal is to dose CYA (using granular stabilizer) to the target level once, and then rely on liquid chlorine to maintain sanitation without adding more stabilizer.
2.3 pH Balancers: The Acid Demand
Control of pH is a weekly, if not bi-weekly, requirement in the summer due to the natural pH drift discussed in Section 1.
- Muriatic Acid (Hydrochloric Acid): This is the industry standard pH reducer for Arizona. It effectively lowers both pH and Total Alkalinity. While hazardous to handle (fuming), it is preferred over dry acid because it does not add sulfates to the water. Sulfates contribute to TDS and can degrade concrete surfaces over time.
- Usage Frequency: In the summer, warm water releases $CO_2$ faster, driving pH up. A typical 15,000-gallon pool in Queen Creek may consume 1/4 to 1/2 gallon of muriatic acid per week to keep pH in the 7.4–7.6 range.
2.4 Algaecides: The Strategic Reserve
In many climates, algaecides are viewed as an optional “up-sell.” In the agricultural interface of San Tan Valley, they are a strategic necessity, particularly for specific resistant strains.
- Polyquat 60: This is a non-foaming, broad-spectrum algaecide that is excellent for maintenance. It works by coating the cell walls of algae, suffocating them and making them easier for chlorine to kill. It is often used as a “safety net” to prevent blooms if chlorine levels accidentally drop.
- Sodium Bromide (The “Yellow” Cure): This is essential for treating Mustard Algae, a chlorine-resistant strain endemic to the region. Products like “Yellow Out” or “Yellow Treat” are technically accelerators; they contain sodium bromide, which, when mixed with chlorine, creates hypobromous acid. Bromine is more lethal to algae than chlorine at high pH levels. This chemical is not for daily use but is a critical tool for eradicating outbreaks.
- Avoid Copper Algaecides: Caution is advised regarding copper-based algaecides. In the high-pH, mineral-rich water of Queen Creek, dissolved copper can easily precipitate out of solution, staining pool plaster and light fixtures a difficult-to-remove turquoise or black.
2.5 Calcium Reducers: The Myth and The Reality
This category is rife with misunderstanding.
- The Myth: Many products are sold as “calcium reducers,” implying they remove calcium from the water.
- The Reality: Chemical agents cannot make calcium atoms disappear. Most “reducers” are actually Sequestering Agents (e.g., HEDP or phosphonic acid derivatives). These chemicals bind to the calcium ions, keeping them in suspension and preventing them from forming scale on surfaces. They do not lower the calcium hardness test reading.
- The Necessity: despite not removing calcium, sequestering agents are vital in Queen Creek. They act as a chemical “inhibitor,” allowing a pool to operate safely with calcium levels of 800 ppm or higher without immediately scaling up the tile line or heater. They are a management tool, not a removal tool.
- True Reduction: The only way to chemically remove calcium is through flocculation (clumping it and vacuuming it out) which is risky and messy, or through physical removal via Reverse Osmosis (RO) or draining.
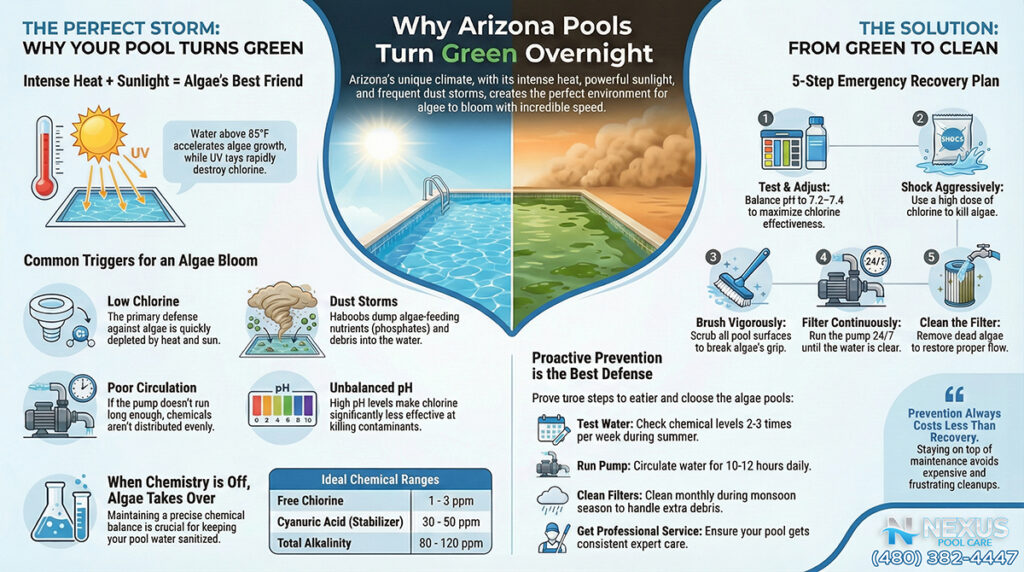
2.6 Phosphate Removers: The Agricultural Defense
San Tan Valley’s agricultural roots present a specific chemical challenge: Phosphates. Fertilizers, windblown dust, and plant debris introduce phosphates into the water.
- The Threat: Phosphates are the limiting nutrient for algae growth. When phosphate levels rise (often exceeding 1,000 ppb in this region), algae can thrive even in the presence of normal chlorine levels.
- The Solution: Lanthanum Chloride is the active ingredient in high-quality phosphate removers (e.g., Orenda PR-10, SeaKlear). It reacts with dissolved phosphates to form a fine precipitate (lanthanum phosphate) that is trapped by the filter. In this specific region, keeping phosphates near zero is a highly effective preventative measure against the aggressive algal blooms fueled by farm dust.
Section 3: Common Chemical Mistakes in the Desert
The harsh environment of the Sonoran Desert is unforgiving. It exposes flaws in pool maintenance routines that might go unnoticed in more temperate zones. Errors in chemical management here do not just result in slightly cloudy water; they can lead to rapid greening, expensive equipment failure, and irreversible damage to pool surfaces.
3.1 Over-Chlorination vs. Wrong-Chlorination
A common misconception is that Arizona pools are “over-chlorinated.” In reality, they are often “wrong-chlorinated.”
- The Stabilizer Trap (Review): As detailed in Section 2, the reliance on Trichlor tabs leads to excessive CYA. The mistake homeowners make is seeing green algae and responding by adding more tabs or shocking with Dichlor (another stabilized chlorine). This adds more stabilizer, worsening the lock. The result is a pool with 10+ ppm of chlorine that still has algae growing on the walls because the chlorine is chemically handcuffed by the stabilizer.
- The “Shock” Confusion: Many homeowners use “Shock” products (often Calcium Hypochlorite) indiscriminately. In Queen Creek, where calcium hardness is already critical, using Cal-Hypo shock weekly adds more calcium to the fire. A 1lb bag of Cal-Hypo in a 10,000-gallon pool raises Calcium Hardness by roughly 7 ppm. Over a summer, weekly shocking can add hundreds of ppm of unwanted calcium. The mistake is not using liquid chlorine for the shock dose.
3.2 Ignoring the Stabilizer (CYA) Ratio
Conversely, some pool owners, fearing “lock,” fail to maintain enough stabilizer.
- The Solar Strip: In June and July, the UV index in Arizona can be extreme. If a pool has 0–20 ppm of CYA, the sun will destroy the free chlorine residual faster than it can be replenished. A homeowner might dose the pool in the morning, and by 2:00 PM, the chlorine is zero. Algae seizes this window to bloom. The mistake is failing to test CYA monthly and maintain that critical 40–50 ppm shield.
3.3 The “Calcium Reducer” Fallacy
Arizona pool owners often fall victim to marketing claims about reducing hardness.
- The Mistake: Pouring bottles of “calcium reducer” into the pool and expecting the hardness test number to drop. When it doesn’t, they add more.
- The Consequence: These products are often acidic or phosphate-based. Overdosing them can plummet the pH, leading to acidic water that etches the plaster, or introduce massive amounts of phosphates (if using older chemistries), which then feed algae. The mistake is not understanding that dilution (draining) or Reverse Osmosis are the only true ways to lower the mineral count.6
3.4 Misdiagnosing Mustard Algae
Mustard algae ($Pleurochloris pyrenoidosa$) is a specific biological threat in the Salt River Valley that is often misidentified.
- The Mistake: Homeowners see yellowish-brown dust on the pool floor or walls (usually on the shady side). They assume it is desert dust or pollen and simply brush it. It disperses into a cloud and disappears, only to reappear in the exact same pattern the next day.
- The Failure: They treat it as “dirt” or standard green algae, shocking it with normal chlorine levels. Mustard algae is highly chlorine-resistant and can survive standard shock levels. It requires the specific sodium bromide protocol (Yellow Out) and the sterilization of all pool toys, vacuums, and swimsuits, as the spores can survive out of water and re-infect the pool. Failure to treat the equipment is the most common reason for recurring mustard algae.
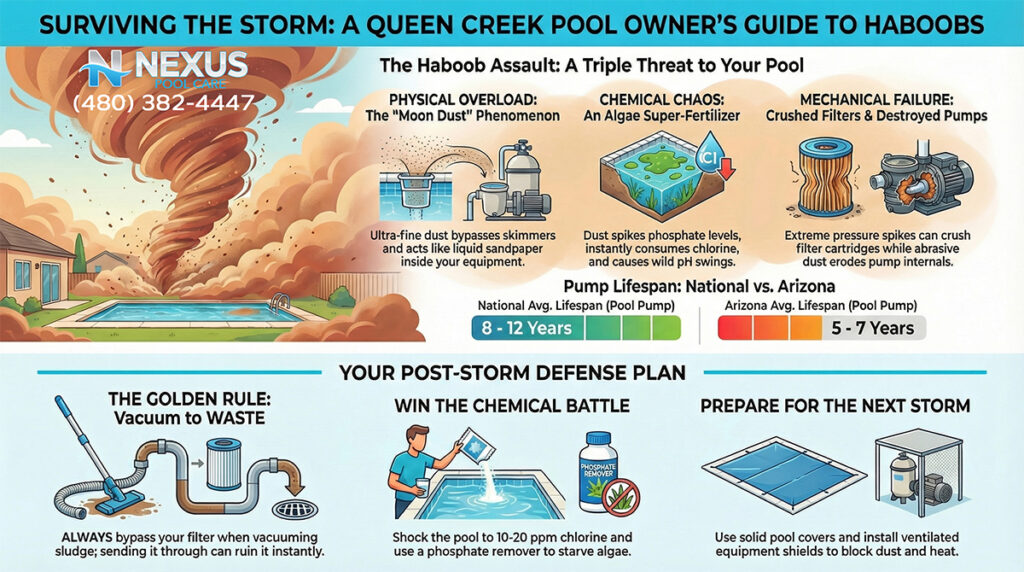
3.5 Post-Monsoon Negligence
The Arizona Monsoon (July–September) is a violent meteorological event that dumps thousands of pounds of particulate matter into pools.
- The Mistake: Waiting for the dust to settle before running the pump, or merely skimming the surface and assuming the filter will handle the rest.
- The Consequence: The dust from haboobs is not just inert dirt; it is laden with organic material, pollen, and phosphates from surrounding agricultural fields. This creates a massive “oxygen demand” and nutrient spike. If the pool is not super-chlorinated (shocked) immediately alongside continuous filtration, the biological load consumes all sanitizer within hours. By the time the homeowner gets around to cleaning the pool two days later, the algae has already established a biofilm, feeding on the new phosphates.
Section 4: Advanced Maintenance Protocols and Professional Optimization
Given the complexity of the variables described—the LSI balance, the agricultural fallout, the extreme heat, and the mineral-heavy source water—pool management in Queen Creek requires a shift from simple “maintenance” to “optimization.” This is where professional methodologies distinguish themselves from typical DIY approaches.
4.1 The Langelier Saturation Index (LSI) Master Class
The difference between a pool that destroys its own surface and one that lasts 20 years lies in the LSI. The Langelier Saturation Index is a calculated number used to predict the calcium carbonate stability of water. It determines whether water will precipitate scale (positive LSI) or dissolve stone/metal (negative LSI).
- The Formula: $LSI = pH + Temperature Factor + Calcium Hardness Factor + Alkalinity Factor – TDS Factor$.
- The Arizona Variable: Temperature is the wildcard.
- Summer: Water temperature hits 90°F–95°F. Heat increases the LSI. A pool with pH 7.8, Alkalinity 100, and Calcium 800 at 95°F has a massively positive LSI (+0.6 or higher), causing rapid scale formation on tile and inside salt cells.
- Winter: Water temperature drops to 50°F. Cold decreases the LSI. That same chemistry at 50°F might drop the LSI to -0.2, becoming corrosive and etching the plaster.
- Professional Optimization: Professionals do not just test pH; they manage the LSI. In the summer, they intentionally run the pH and Alkalinity lower (pH 7.4, Alkalinity 80) to counteract the heat and calcium, keeping the LSI near 0.0. In the winter, they raise the pH and Alkalinity to prevent the cold water from eating the pool surface. This dynamic adjustment is the key to longevity in the desert.
| Season | Water Temp | Chemical Strategy | Target LSI |
| Summer | 90°F – 95°F | Suppress pH (7.2-7.4) and Alkalinity (70-80) to prevent scale. | -0.3 to +0.3 |
| Winter | 50°F – 60°F | Elevate pH (7.6-7.8) and Alkalinity (100-120) to prevent etching. | -0.3 to +0.3 |
4.2 Precision Testing and Cost Control
While professional service involves a monthly fee, it often offsets the “hidden costs” of chemical mismanagement.
- Testing Technology: Professionals utilize titration-based test kits (like the Taylor K-2006) or digital photometers (SpinTouch) that offer precision down to 0.2 units of pH and 10 ppm of Calcium. This contrasts with test strips, which can be vague and lead to overdosing or underdosing.
- Chemical Efficiency: By calculating exact dosages using the LSI, professionals avoid the “dump and pray” method. They use industrial-strength liquid chlorine (12.5%) which is more cost-effective per unit of active sanitizer than the diluted products found in big-box stores.
- Preventing the Drain: As noted, the greatest cost in Arizona is draining the pool due to runaway CYA or TDS. By using non-stabilized chlorine and managing TDS, professionals can extend the life of the water by years, saving hundreds of dollars in water bills and startup chemicals.
4.3 Seasonal Adjustments: The Monsoon Protocol
Professional optimization includes a specific operational posture for the monsoon season (July–September).
- Pre-Emptive Strike: Before the season begins, professionals often dose the pool with a phosphate remover to lower the nutrient floor. They may also add a borate preventative or a polyquat algaecide to create a “sanitary buffer.”
- Storm Response: The protocol shifts to “all hands on deck.” Filter run times are increased to 12-18 hours per day to scour the water. Chlorine levels are maintained at the higher end of the range (4-6 ppm) to handle sudden organic loads. Baskets are emptied daily to prevent flow restriction that could overheat the pump.
4.4 Advanced Interventions: Reverse Osmosis (RO)
When chemical management reaches its limit—typically when Calcium Hardness exceeds 1,000 ppm or Cyanuric Acid exceeds 100 ppm—professionals in the Queen Creek area have access to advanced remediation: Mobile Reverse Osmosis.
- The Process: A massive, trailer-mounted industrial RO unit parks in front of the home. It pulls water from the pool, pushes it through high-pressure membranes to strip out calcium, salts, metals, and CYA, and returns drinking-quality water to the pool.
- The Advantage: This process conserves up to 85% of the existing water, a critical consideration in Arizona. It allows the pool owner to “reset” their water chemistry to near-perfect conditions (low hardness, zero CYA) without the risk of exposing the plaster to the harsh desert sun and heat, which can crack a drained pool shell.
The Bottom Line: Balanced Water Saves Money and Protects Your Pool
For the residents of Queen Creek and San Tan Valley, the swimming pool is an essential component of the lifestyle, a necessary refuge from the oppressive heat. However, the geology and climate of the region conspire to degrade this asset through scaling, corrosion, and biological attack.
The “best” pool chemicals for this region are not found in a single magic bottle. They are a specific class of compounds chosen to counteract the local hostile environment:
- Liquid Chlorine to provide powerful sanitation without the structural penalty of stabilizer or calcium buildup.
- Muriatic Acid to aggressively manage the high-pH, scale-forming nature of the aquifer.
- Phosphate Removers to neutralize the specific nutrient pollution from the surrounding agricultural zone.
- Sequestering Agents to act as a chemical shield against the inevitable hardness of the water supply.
By shifting from a reactive mindset—treating the pool only when it looks dirty—to a proactive, science-based mindset that manages the LSI and TDS, pool owners can ensure their investment remains a sparkling oasis rather than a calcified, green liability. Whether managed via diligent DIY efforts or through the expertise of a professional service, the key to success in the Arizona desert is a healthy respect for the unique, dynamic, and powerful chemistry of the water.
Technical Appendix: Chemical Parameter Targets for Arizona
The following table summarizes the optimized chemical targets for a plaster pool in the Queen Creek/San Tan Valley area, adjusting for the high heat of summer.
| Parameter | Ideal Range (National) | Arizona Summer Target | Rationale | Chemical to Adjust |
| pH | 7.4 – 7.6 | 7.2 – 7.4 | Lower pH offsets the high LSI caused by 90°F+ water and high calcium, preventing scale. | Lower: Muriatic Acid
Raise: Soda Ash (Rarely needed) |
| Free Chlorine (FC) | 1.0 – 3.0 ppm | 3.0 – 5.0 ppm | Higher residual required to combat intense UV burn-off and algae growth rates. | Raise: Liquid Chlorine (12.5%) |
| Total Alkalinity (TA) | 80 – 120 ppm | 70 – 90 ppm | Lower alkalinity helps keep pH lower and reduces the “pH ceiling,” managing LSI. | Lower: Muriatic Acid
Raise: Sodium Bicarbonate |
| Calcium Hardness (CH) | 200 – 400 ppm | 250 – 500 ppm | Managing high calcium is the norm; rely on LSI balance rather than trying to lower CH chemically. | Lower: Drain/Refill or RO
Raise: Calcium Chloride (Rarely) |
| Cyanuric Acid (CYA) | 30 – 50 ppm | 40 – 50 ppm | Essential for UV protection, but strictly capped to prevent chlorine lock. | Lower: Drain/Refill
Raise: Stabilizer Granules |
| Phosphates | < 100 ppb | < 100 ppb | Zero tolerance due to agricultural dust and resistant algae strains. | Lower: Lanthanum Chloride |
| Total Dissolved Solids | < 1500 ppm | < 2500 ppm | High evaporation raises baseline; monitor for “tired water” symptoms. | Lower: Drain/Refill or RO |
Reference Integration
This report synthesizes data from the Town of Queen Creek Water Quality Reports 1, hydrogeological analyses of the Salt River Valley 8, and technical guidelines from pool chemistry experts regarding the LSI and high-temperature management. Specific product efficacy data regarding algaecides and phosphate removers 3 and economic comparisons of chlorination methods 19 were integrated to provide a comprehensive operational guide